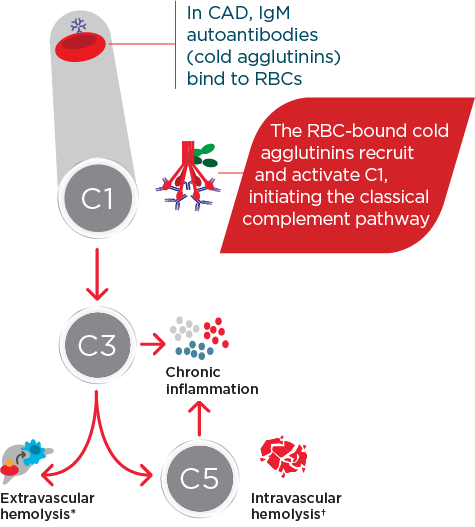
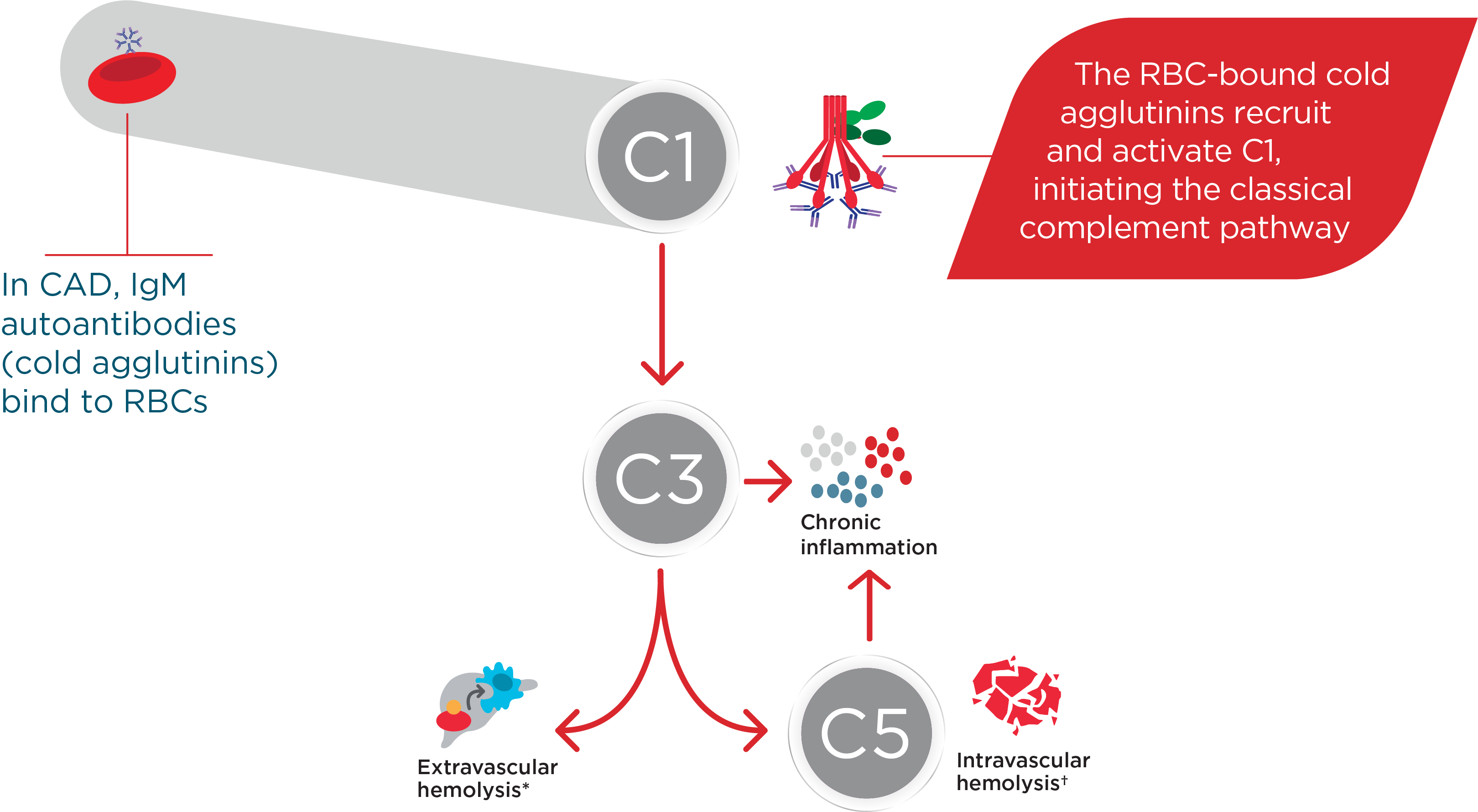
Hi, I am Dr Catherine Broome and we are here today to discuss the risks associated with C1 activated hemolysis in Cold Agglutinin Disease. This presentation is being sponsored by Sanofi and I am being compensated by Sanofi for my participation. We are going to start the day with an introduction to Cold Agglutinin Disease. We are then going to discuss some of the mechanisms of how Cold Agglutinin Disease develops. We are going to delve into some of the very serious and significant risks associated with Cold Agglutinin Disease as well as the disease burden and we are going to wrap up with some concluding remarks about this disease and some of the very serious complications that can occur for these patients. So, Cold Agglutinin Disease is a serious autoimmune hemolytic anemia. It has systemic, both acute and chronic, consequences. It is a chronic condition, characterized by autoimmune mediated destruction of red blood cells. These patients are chronically compromised and vulnerable to hemolytic crises. The disease itself also lends an increased risk to the development of thromboembolic events. The hemolysis in this disease is entirely dependent on activation of the classical pathway of complement. About 20% of all patients with autoimmune hemolytic anemia have Cold Agglutinin Disease. It is a rare disorder, it affects approximately 16 people per million of the population and the average age of onset in these patients is about 58 years; although, the disease can occur as early as 30 years of age. So, unlike cold agglutinin syndrome, Cold Agglutinin Disease is not secondary to any underlying malignancy or infection. Cold agglutinin disease is defined as chronic, autoimmune hemolytic anemia. It is classical pathway–dependent hemolysis, and there is a B-cell expansion that is nonprogressive and clinically nonmalignant. In contrast, cold agglutinin syndrome is also a hemolytic anemia, but it is secondary to overt malignant disease or secondary to an infection. The distinction between Cold Agglutinin Disease and cold agglutinin syndrome is quite important as we think about managing these patients. Thinking about the diagnosis of Cold Agglutinin Disease is the most important step. It requires often laboratory evaluations that are not part of our immediate panel that we do when we evaluate patients with anemia. It requires the presence of both a positive direct antibody test as well as the presence of IgM autoantibody. We want to look for C3d on the surface of the red blood cells through a direct antiglobulin test, and we also want to look for the presence of this IgM antibody by testing cold agglutinin titers. Once we have established that the IgM antibody is present and that there is in fact C3d on the surface of our red blood cells, we want to further evaluate our patients, ruling out the likelihood that this could be a secondary or cold agglutinin syndrome, related either to infection or to overt malignancy. Some key identifiers that I use when thinking about Cold Agglutinin Disease would certainly be evidence of a hemolytic anemia. As we are evaluating anemia in any patient, we want to specifically look at LDH, haptoglobin, bilirubin, as well as a reticulocyte count. Many of these patients can have a well-compensated hemolysis, which may only be evident to us if we see a significant elevation in their reticulocyte count. Once we see evidence of hemolysis, the next step is going to be to perform a direct antiglobulin test. The antiglobulin test is going to let us know whether this is a hemolysis that is mediated by IgG or a warm antibody, or whether it is a hemolysis that is specifically mediated by complement, in which case, the direct antiglobulin test will have C3d positivity. This is our clue that there are circulating IgM antibodies in the patient’s plasma and the next step that we should take is to evaluate the titer of these IgM antibodies through a cold agglutinin titer. …If you do not do these steps, you may miss the diagnosis of Cold Agglutinin Disease. So, the disease course of Cold Agglutinin Disease can be very unpredictable. It has been previously written that this is a fairly indolent or benign disease. As we learn more about Cold Agglutinin Disease, we are certainly discovering that most patients’ journey with this disease is anything but indolent or anything but benign. There can be an array of symptoms and an array of signs that these patients do present with, including shortness of breath, fatigue…These patients may be clinically jaundiced. They may also complain of darkening of their urine related to the hemolysis. They may also complain of symptoms of pain in their extremities, including their fingers and their toes, related to acrocyanosis from agglutination of red blood cells, secondary to the IgM antibody that is circulating in their plasma. So, a typical patient journey to diagnosis often takes many visits to a variety of healthcare providers. These patients present with very nonspecific symptoms. They are tired, they have no energy, they may be short of breath, they may complain of feeling like their hands and their feet are cold all the time. As you can see, this constellation of symptoms may represent a variety of diagnoses, particularly in an older population. These patients often have symptoms for many years before they receive the appropriate testing—looking for the antibodies on the surface of their red blood cells, the complement deposition, and the antibodies in their serum, the cold agglutinin. I think the most challenging aspect for patients and their journey is that their symptoms are so nonspecific, that they are often dismissed as symptoms of getting older, or symptoms of not exercising enough, or symptoms of, you may be a little bit depressed, or you may not be sleeping well. I think we need to listen carefully to our patients and perform a thorough evaluation so that we can recognize and diagnose these patients as early as possible. Markers of active hemolysis indicate ongoing risk for patients with Cold Agglutinin Disease and they include an elevated bilirubin which suggests extravascular hemolysis Cold Agglutinin Disease and LDH, which may be elevated as well, which indicates red blood cell destruction as well as—we mentioned previously—an elevated reticulocyte count. Patients are relatively healthy, and they have healthy bone marrow; the response to the anemia is going to be to generate more red blood cells, which we can measure by looking at this reticulocyte count. So, how does this disease develop? What is the actual pathophysiology? In Cold Agglutinin Disease, a clonal B-cell expansion produces a monoclonal IgM autoantibody. These IgM autoantibodies, because their activity is dependent on a cooler than body temperature, are called "cold agglutinins." So, as you can see in this diagram, native B-cells will expand clonally. They will produce this IgM antibody or cold agglutinin and this cold agglutinin will bind to antigens on the red cells surface. Once that occurs, it leads to agglutination, or sticking together of these red blood cells. It also leads to activation of the classical complement pathway. Now we are going to talk about complement and complement activation. As you will recall, there are 3 distinct pathways that lead to complement activation. Normally, these pathways converge at C3 to destroy pathogens. In the classical pathway, the C1 complex is activated by the binding of an antigen-antibody complex. In the lectin pathway, various lectin complexes activate by binding sugars on pathogens and, in the alternative pathway, specific factors activate upon spontaneous hydrolysis of C3 on pathogens. The classical and lectin pathways cleave C2 and C4 to form C3 convertase. The alternative pathway relies on separate factors to form C3 convertase. Once active, C3 convertase initiates the complement response. Cleaved active products of C3 and C5 drive the effector responses. C3b coats pathogens for phagocytosis. C5b along with C6 through C9 form the membrane attack complex, which creates holes in the pathogens cell membrane, resulting in lysis. C3a and C5a are potent inflammatory regulators and interact with many cell types of the immune system, further driving the inflammatory response that we associate with complement activation. While all 3 of these pathways converge at C3, it is important to remember—and we will discuss further—that the hemolysis associated with Cold Agglutinin Disease is specifically mediated by the classical pathway driven by the activation of the C1 complex by the antigen-antibody interaction. In CAD, activated C1 really drives this hemolysis entirely through the classical pathway and you can see in this diagram, as we just discussed, that C1 activation leads to C3 cleavage. C3 is deposited on the surface of these red blood cells. We measure that with the direct antiglobulin test, and as these cells circulate through the patient’s they are recognized and cleared from the circulation by a process called opsonization. Some percentage of these cells may go on to be destroyed with the terminal portion of complement activation through the membrane attack complex. In general, the membrane attack complex is not generated in a particularly high number of patients with Cold Agglutinin Disease. The vast majority of the hemolysis is in fact extravascular opsonization of C3 coded red blood cells. So, what happens to these patients? Yes, they are anemic. Yes, they are tired. Can we not just give them a blood transfusion and make them feel better? The truth of the matter is that these patients’ lifestyles and their overall health is very severely affected by Cold Agglutinin Disease. Depending on the severity of the disease, the management strategies that we have had for patients includes some very nonspecific approaches. We can tell patients to try not to get cold, because we know that that activates the IgM antibody. We can intervene in a crisis, when they are profoundly anemic—we can give them transfusions and there are some pharmacotherapies that have been tried for these patients. But currently there are no approved therapies for Cold Agglutinin Disease. We are left with symptom management. The biggest unmet need or challenge for treating Cold Agglutinin Disease, really, is a therapy that can be specifically directed to the pathophysiology mechanism. We can find an appropriate therapy that will inhibit the hemolysis, inhibit complement activation, inhibit agglutination of these red blood cells. We can absolutely improve the quality of life and hopefully also improve morbidity and mortality for these patients. I think there are many challenges that the patients see. First of all, education of the healthcare community in general about Cold Agglutinin Disease. What are the signs and symptoms? How do we diagnose these patients earlier? Then how do we get our patients the best possible care that is currently available and all of these things require a high level of suspicion that a patient might have this very rare disease before we can embark on the pathway of actually helping benefit our patients with Cold Agglutinin Disease. So, in spite of some non-specific approaches, these patients are in a constant hemolytic state. I think there are some misconceptions that this is a disease that comes and goes. If we look very carefully at data in Cold Agglutinin Disease patients, we see that they chronically exhibit evidence of ongoing hemolysis, whether they are compensated or not. Their bilirubin levels are consistently above normal, their LDH levels are also consistently above normal. 73% of patients that are represented in these slides actually received some of the currently available, but non-approved therapies for Cold Agglutinin Disease over a median follow-up time of 5 years. I think these data demonstrate that our current approaches have been less than optimal for providing patients relief of the chronic hemolysis that is associated with Cold Agglutinin Disease. These patients are at risk for developing severe hemolytic crisis at any time and these crises can be very unpredictable. They may be triggered by events that seem very clinically nonsignificant: a mild upper respiratory infection, a mild viral gastroenteritis, stress, minimal exposure to changes in temperature. In a retrospective review of a healthcare system database in patients with Cold Agglutinin Disease, among all patients with Cold Agglutinin Disease, 72% of these patients had one or more severe anemia event within the first year of follow-up and 67% of these patients had a severe anemia event within 6 months of starting these nonspecific therapeutic interventions that we currently have available. With our current management strategies, we have noticed that these patients still require and utilize extensive healthcare resources. This study found that within 1 year after diagnosis of CAD, these patients require an extensive healthcare resources compared to the general population cohort. 2.5 times more patients with CAD use hospital inpatient services. Patients with CAD required an average of 17.3 outpatient visits compared with 6.7 visits for matched comparisons, and 1.5 more patients with CAD required emergency room visits compared with marked comparisons. There are some limitations to this study as well, patients were only selected from the Optum-Humedica dataset and patients with CAD were identified using clinical notes, not diagnostic codes or laboratory evaluations. Some people feel that if you move to a warmer climate or you avoid going out in the winter time, you can avoid some of the symptoms associated with Cold Agglutinin Disease. In a recently published trial looking at seasonality in Cold Agglutinin Disease, the burden of the disease is very consistent, irregardless of the season. So, the number of inpatient hospitalization days, the number of outpatients visit days was no different when we compared fall to summer, winter to summer, or spring to summer. So, this is a disease that is active 24 hours a day, 365 days a year for patients with Cold Agglutinin Disease. A typical day for cold agglutinin patients is, I think, fraught with making decisions. They are very fatigued, they oftentimes have to decide what it is that they are going to spend their energy on that day, what it is they are going to spend their energy on that week. So, they really are in a position where they have to modify their lifestyle. Many of them are retired and looking forward to enjoying travel or other activities with their loved ones, with their spouses, with their families, with their friends; but they are forced, because of the fatigue, the shortness of breath, the concern for whether or not they will have a crisis while they are away from their healthcare team, to make some very difficult decisions on what they feel comfortable doing on any given day. It is unfortunate that these patients are forced with these decisions on a regular basis. So, some of the serious risks that are associated with Cold Agglutinin Disease include an increased risk in thromboembolic events. There was a 55% increase in patients with Cold Agglutinin Disease compared to a matched comparison group of patients with the same age and the other same comorbidities. We conclude that it is the Cold Agglutinin Disease itself, the hemolysis, the complement activation that are all contributing to this increased thromboembolic risk. Many of these patients go undiagnosed with regards to their thromboembolisms. I think symptoms of pulmonary emboli—shortness of breath, dyspnea on exertion—are oftentimes over looked in a population of elderly patients or patients with anemia. These symptoms may be attributed to the anemia itself; but now that we recognize that this is a risk in these patients, I think as healthcare providers we must be more attuned to evaluating these patients thoroughly for the possibility that some of these symptoms may be related to embolic phenomena. A significantly higher incidence of more than 1 type of thromboembolic event was also seen in patients with Cold Agglutinin Disease. So, these patients are having multiple issues as they are dealing with their Cold Agglutinin Disease compared to an age and comorbidity-matched control population. These events are not occurring because the patients are elderly, they are occurring because they have an underlying hemolytic, chronic complement activating disorder. They have an increased risk of potentially fatal thromboembolic events as well. When you look at comparison cohorts, these patients had an increased risk of strokes, of myocardial infarctions, as well as pulmonary emboli, all of which can lead to early mortality and certainly significant morbidity for this population of patients. How do we predict who is at risk for developing a thromboembolic phenomenon in our patients with Cold Agglutinin Disease? Interestingly, we had initially expected that perhaps those patients who were more severely anemic, related to a higher level of hemolysis and a lower hemoglobin, may be more at risk. When we looked at the data, what we saw in patients that had a thromboembolic event was that 1 month prior to that event, the vast majority of patients had what would be characterized as mild anemia. The degree of anemia did not seem to be a direct predictor of thromboembolic risk. Conversely, markers of ongoing hemolysis including an elevated LDH, an elevated bilirubin were both associated with thromboembolic risk and over 90% of patients who had a thromboembolic event had one or both of these markers of ongoing hemolysis. So, active hemolysis may really be the predictor of thromboembolic risk, regardless of degree of anemia. Correlation between complement activity, hemolysis, and serious risks for patients with CAD—I think we have gained some understanding about the interrelationship of these issues in regards to patients who have another complement mediated hemolysis, which is paroxysmal nocturnal hemoglobinuria. Activation of complement not only is related to hemolysis but can also be directly related to activation of the coagulation cascade, endothelial injury, activation of platelets, which will all come together to increase a patient’s risk of developing thromboembolic complications. I think our understanding of the pathophysiology of Cold Agglutinin Disease and what that pathophysiology can do in its interactions with other systems within coagulation, platelet activation, and endothelial cell health will go a long way to helping us understand the mechanism of thrombosis in patients with Cold Agglutinin Disease. When we look at patients with Cold Agglutinin Disease and we compare them to age-matched controls in the general population, they in fact do have increased mortality. This study looked at mortality in Cold Agglutinin Disease and we used a Kaplan-Meier evaluation to compare cold agglutinin patients to aged matched controls. They were identified in the Danish National Patient Registry from 1999 to 2013 and these patients were matched based on age, sex, region, and adjusted for comorbidities. And you can see that 61% of patients with CAD were likely to be alive at 5 years compared to 82% of the matched cohort; and as you look at these curves in this Kaplan-Meier analysis, you can see that this increased risk appears as early as 1-year post diagnosis of Cold Agglutinin Disease. Mortality is increased in patients with primary CAD, regardless of cancer status. Previously there had been some postulation that, yes, these patients have a monoclonal B-cell population and therefore their risk of mortality may be associated with the progression of that to a more malignant form of lymphoma or other cancers. When we looked at this analysis using Cox regression analysis and calculated hazard ratios, the sensitivities were conducted, we excluded patients with other diagnoses and there was a 3 times increased mortality risk within the first 5 years after diagnosis of Cold Agglutinin Disease. While these mortality data are intriguing, we definitely need additional studies to further evaluate the effect of Cold Agglutinin Disease on mortality. Overall, this disease is a high burden and contributes to serious risk in these patients. It is the ongoing complement activation and the chronic hemolysis that are the main contributors to the disease burden and the serious risks that these patients encounter associated with their diagnosis. In addition to those health risks, there is absolutely an increased risk of healthcare utilization and an increased risk of morbidity and mortality associated with this disease. So, I think my approach to these patients has definitely evolved over the last 3 to 5 years. Diagnosing Cold Agglutinin Disease in the past tended to be an exercise in academic curiosity. We did not really have any specific therapeutic interventions to offer these patients. We could transfuse them when they needed transfusions. It was more of an oddity and less of a diagnosis that we sought to try to find in these patients. As we have learned more about complement and complement’s role in the disease burden for these patients, I think the onus now falls to healthcare providers to be aware of this disease, to be aware of the appropriate diagnostic testing in order to identify these patients. Our awareness of the increased risk of thromboembolic phenomenon, I think, makes all of us accountable to evaluate these patients thoroughly for signs and symptoms that may also be attributed to thromboembolic disease. We need to treat these patients aggressively if we do encounter thromboembolic diagnoses and hope that on the horizon, we will have additional potential therapeutic interventions for those patients in whom we have made this diagnosis. So, Cold Agglutinin Disease is a very chronic condition. It can have incredibly severe consequences for our patients. It is mediated and driven by C1 hemolysis. There are the potential for many serious risks for these patients, including hemolytic crisis, thromboembolic phenomenon, increased morbidity, and increased mortality. And at the present, there are no approved therapeutic interventions. We manage symptoms in these patients, we try to educate ourselves as far as the risks of other phenomenon that can be associated with this disease, and we continue to watch for forthcoming potential therapeutic interventions. I think some of the biggest misconceptions about Cold Agglutinin Disease are that it is an indolent, benign form of anemia that does not require consistent monitoring, does not require a high degree of suspicion of other complications that may occur with it. I think also a misconception is that this does not affect patients’ lives. It certainly affects their daily lives and as we are now learning from some of these more recent studies that we previously discussed, it also affects their longevity. It affects their mortality and when a disease is so serious that it affects both the patients’ daily life as well as their overall mortality, I think it is incumbent upon us to make it our job to recognize and diagnose these patients, so that we can, as their healthcare providers, guide them through this journey as safely as possible. Thank you for your time and attention this afternoon.